

We recently published an overview of the MedTech and BioTech innovation cluster at High Tech Campus Eindhoven.
This article, the third in the series, highlights companies developing implants and therapeutic devices, smart biomaterials, sports, vitality and health and research institutions located at HTCE.
The first article introduced the 70+ organizations fundamentally shaping healthcare innovation at HTCE.
The second article showcased the innovations that make this ecosystem so important and so powerful, including companies developing robotic precision systems, intelligent diagnostics, continuous monitoring wearables and AI-driven software.
Our timing on publishing this series about the MedTech and BioTech ecosystem at HTCE couldn’t be better.
In his December 2025 report “The Road to Future Prosperity,” former ASML CEO Peter Wennink highlighted biotechnology as a key tech domain for Dutch competitiveness. He projects it could create 20,000 jobs and add 1.2 percentage points to GDP, with each public euro attracting €2.50 in private investment. Wennink calls for €150 billion in investment over the next decade to keep pace with the U.S. and China in strategic sectors.
We couldn’t agree more.
There are many opportunities to investment in companies at HTCE, which has become the launchpad for MedTech and BioTech innovations defining what’s possible in healthcare.
Here, a powerful ecosystem of innovators is achieving medical “firsts” that will shape the future: implants that restore function without drugs, materials that guide the body to heal itself and preventive technologies that keep people healthy and active throughout their lives.
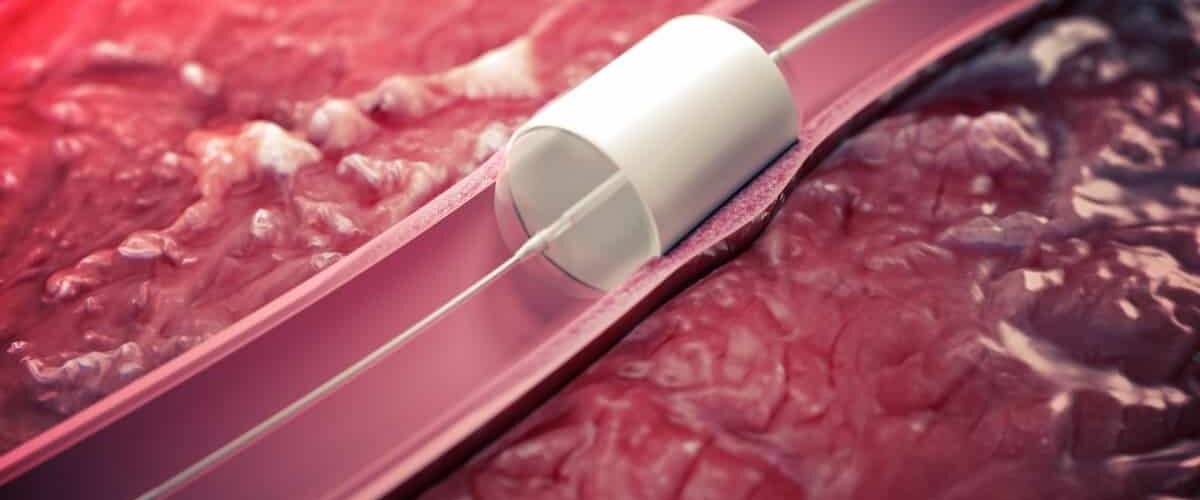
For example, STENTiT delivers minimally invasive cardiac interventions, and Salvia BioElectronics is testing its thin implant for people with migraine, offering drug-free pain relief using neuromodulation.
They’re thriving because they’re part of something bigger.
Campus MedTech and BioTech companies leverage leading research institutes such as Holst Centre, imec and TNO, who is making fundamental discoveries in flexible electronics, wearable sensors and advanced diagnostics and turning them into commercially viable solutions.
HTCE residents draw on critical infrastructure such as the Smart Biomaterials Consortium’s GMP-compliant cleanroom facilities, which bridges the gap between life-changing product concepts and market-ready medical devices.
This is what makes HTCE such fertile ground for Medtech and BioTech companies. It's a complete, verdant ecosystem where collaboration accelerates innovation on the way to scientific breakthrough and, ultimately, better patient outcomes and a healthier society.

Download the entire overview of the more than 70 MedTech & BioTech-focused companies at HTCE.
Implants and therapeutic devices represent a paradigm shift from managing symptoms to actively restoring function and promoting healing within the body. These advanced technologies range from bioelectronic implants that deliver drug-free pain relief through precise nerve stimulation to bioresorbable scaffolds that trigger natural regeneration before harmlessly dissolving.
By combining sophisticated materials science with deep understanding of biological processes – from cold plasma therapy that accelerates wound healing to minimally invasive cardiac interventions – these devices offer patients less invasive alternatives to traditional treatments while achieving superior clinical outcomes.
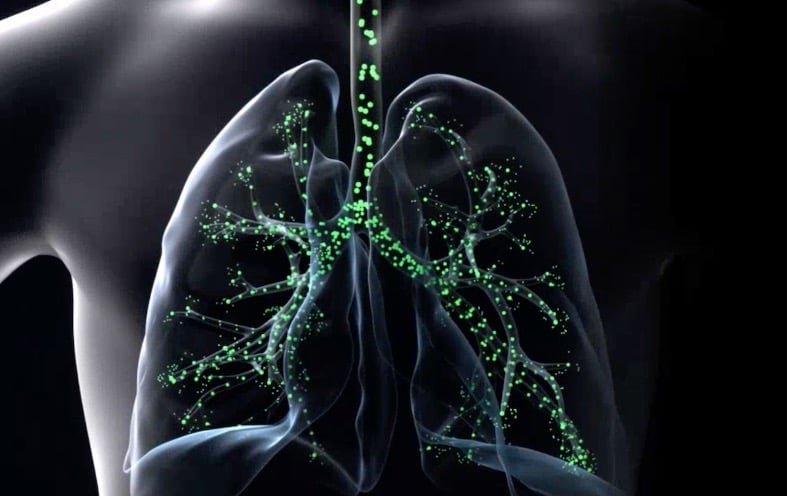
Illustration credit: Gilbert
Gilbert develops advanced medical inhalation devices for lung patients using Electro Hydrodynamic Atomization (EHDA) technology. Gilbert’s breath-actuated smart precision inhaler creates a monodispersed soft mist aerosol with customizable droplet size for precise drug targeting to desired lung regions. Gilbert, a Delft University of Technology (TU Delft) spin-off, focuses on treating cystic fibrosis, asthma, lung cancer and other respiratory diseases.
Gilbert was a 2023 recipient of the Gerard and Anton Award and closed a €7M seed and Series A funding round the same year.
Photo credit: Jenscare Scientific
Jenscare Scientific, headquartered in Ningbo, China, is a medical device company that develops innovative solutions for structural heart disease. Since its launch in 2011, Jenscare has developed minimally invasive interventional medical devices in the field of structural heart disease to fulfill the large but underpenetrated market. Covering tricuspid valve disease, mitral valve disease, aortic valve disease, heart failure and cardioembolic thrombi prevention, Jenscare has a comprehensive and advanced product portfolio for structural heart diseases. Its core product, Lux-Valve Transcatheter Tricuspid Valve Replacement System, is expected to be one of the first TTVR products approved for commercialization globally.
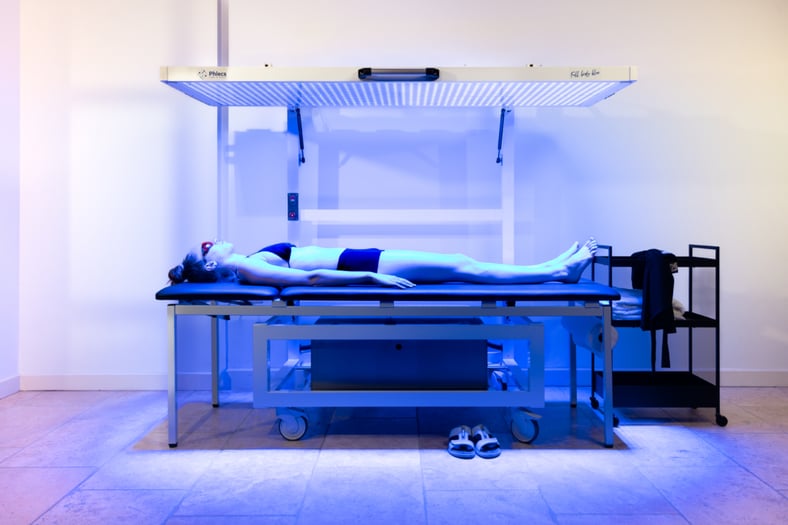
Photo credit: Phlecs
Phlecs is a startup that leverages 15 years of clinical and technology research originated at Philips Healthcare. Phlecs develops and commercializes photo-dermatology solutions, offering a gentle, safe, and clinically proven Blue LED Therapy device for treating inflammatory skin conditions such as psoriasis, eczema and atopic dermatitis.
Phlecs’ non-invasive treatment uses blue light to kill bacteria, reduce inflammation and stimulate collagen production. UV-free blue light therapy offers a safe, effective alternative to traditional UV phototherapy, which will be phased out with the EU ban on fluorescent lamps in February 2027.
The timing of this ban makes Phlecs’ technology particularly relevant as full-body blue LED therapy emerges as a promising and accessible treatment option.
The company recently received a €1 million grant from the OP-Zuid Stimulus program to develop whole-body blue light devices that will allow patients to be treated at home, with Phlecs leading the project on market access preparation alongside several partners.
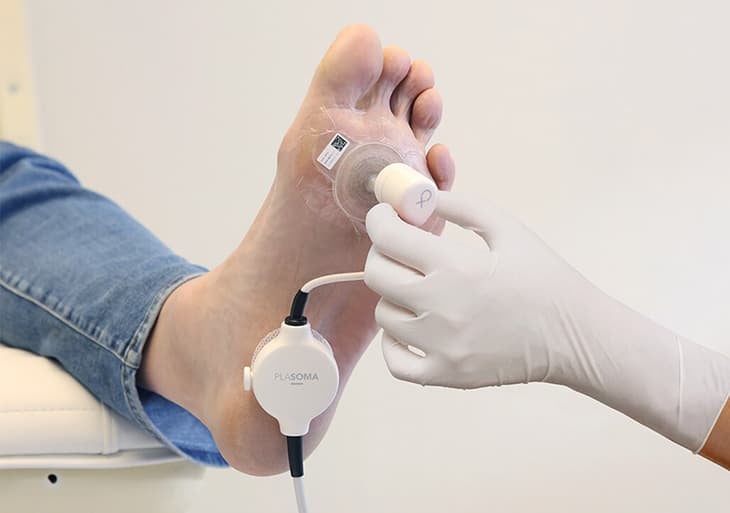
Photo credit: Plasmacure
Plasmacure is a MedTech startup founded in 2014 by Bas Zeper. Leveraging the extensive expertise in cold plasma generation at TU/e, Zeper and fellow researchers developed PLASOMA, an innovative medical device that applies cold plasma therapy directly to chronic wounds to promote healing.
The system consists of a cold plasma pad placed on the wound and connected to a pulser that generates energy to create the therapeutic cold plasma. Clinical trials conducted on diabetic foot ulcers show promising results, with 11 of 20 wounds reducing by more than 50% in size within two weeks and other wounds healing completely. The treatment also significantly decreased harmful Staphylococcus aureus colonies immediately after plasma application. PLASOMA is CE-certified.
The company has achieved significant recognition and funding milestones, including winning the Gerard & Anton Award in 2017 and securing a substantial €6 million funding round in July 2025. This strategic investment, led by U.S.-based Venture Medical LLC and other partners such provides direct funding and more than $10 million in U.S. go-to-market financing. The funding supports the international rollout of PLASOMA, positioning the revolutionary cold plasma therapy for broader market penetration in treating complex wounds.
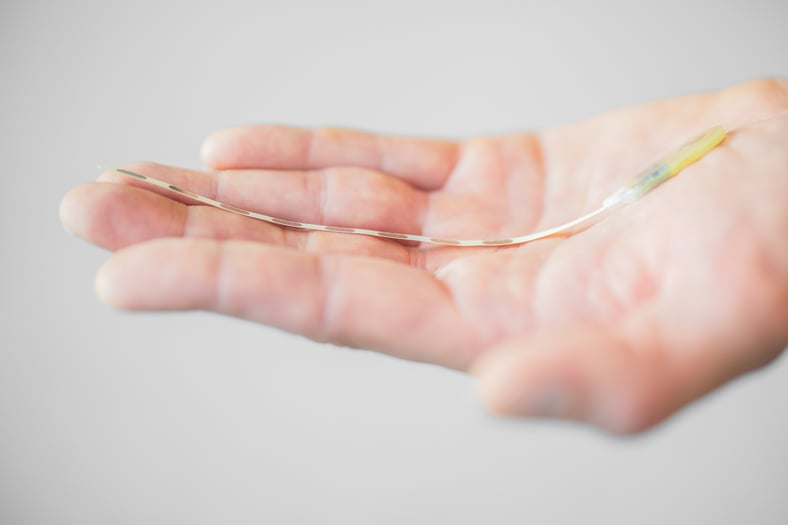
Photo credit: Salvia BioElectronics
Salvia BioElectronics, a MedTech scale-up, developed neuromodulation therapy for people with chronic migraine. It is an ultra-thin implantable device activated with a simple tap on a small wearable device. Called “MySalvia Therapy,” it consists of two ultra-thin implants: one placed just beneath the skin of the forehead and another just beneath the skin at the back of the head. The bioelectronic foils target key nerves to provide drug-free relief, representing a minimally invasive alternative to traditional neurostimulation systems.
Hubert Martens, Wim Pollock and Daniel Schobbens co-founded Salvia in 2017. In May 2025, Salvia completed a $60 million Series B financing round, led by Innovation Industries (HTC 41), to accelerate the development and commercialization of its therapy. Clinical trials are underway in Australia and Belgium, with the first patient in the Netherlands receiving the implant in February 2025 as part of a blinded, sham-controlled study. The Series B funding will help Salvia complete clinical trials and take its technology to market, with the goal of helping millions of people with chronic migraine reclaim their lives.
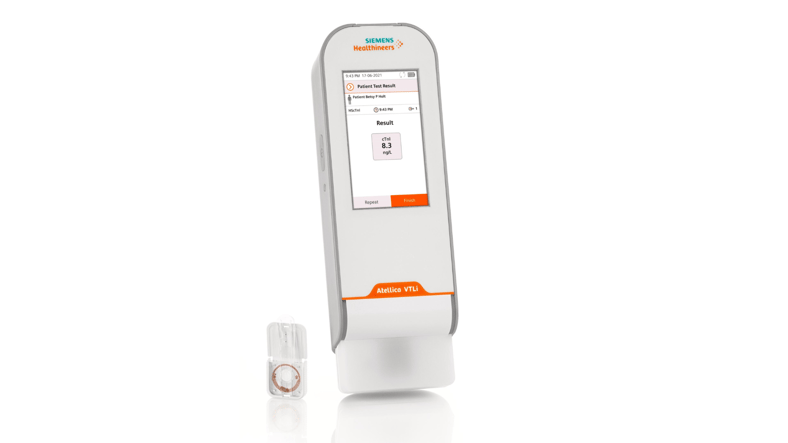
Siemens Healthineers is focused on point-of-care cardiac testing. Imagine going to the hospital with symptoms of a heart attack, only to wait an hour for the test results and treatment. With each passing minute, your health is in jeopardy. Siemens Healthineers set out to change this scenario in emergency departments across the world.
At HTCE, the team has developed a handheld device called Atellica® VTLi Patient-side Immunoassay Analyzer. While it’s easy to operate, the microfluidics technology inside is cutting-edge. Filtered plasma from a drop of blood goes into reaction chambers with magnetic particles which capture the troponin. These particles are manipulated and the device quantifies the amount of troponin. This technology provides lab-quality cardiac immunoassay testing at the right time and setting for patients.
Siemens Healthineers Eindhoven is part of Siemens Healthineers' broader strategy to push boundaries in personalized medicine, precision therapy and digital health solutions, all to improve healthcare access and outcomes sustainably.

Smart biomaterials represent the convergence of materials science and biology, creating substances that don't just replace damaged tissue but actively guide the body's own healing processes.
These next-generation materials – from electrospun nanofibers that mimic the body's natural extracellular matrix to bioresorbable scaffolds that provide temporary support before disappearing – are designed to work in harmony with human physiology rather than as permanent foreign objects.
The establishment of GMP-compliant cleanroom facilities at High Tech Campus Eindhoven addresses a critical infrastructure gap, providing MedTech companies with the specialized manufacturing environment needed to transform these innovative biomaterials from laboratory concepts into market-ready medical devices.

SBMC Director Jan Rietsema. Photo credit: SBMC
Smart BioMaterials Consortium (SBMC) provides high-quality facilities for the industrialization of smart biomaterials and biodegradable implants used for regenerative medicine-based technologies.
For the MedTech sector, SBMC solves a critical commercialization bottleneck by offering flexible, high-spec cleanrooms for pilot production of medical devices, biomaterials and implants through a new GMP cleanroom facility. This facility, opening mid-2025, provides MedTech companies with ready-to-use, compliant cleanroom rental options, eliminating the need to build their own expensive facilities.
Beyond its coveted cleanrooms, SBMC strengthens the regional ecosystem through strategic partnerships. In December 2024, SBMC signed agreements with HTCE, TU/e, and other regional partners to position Brainport as the key international hub for smart biomaterials development. Its €4M investment in shared manufacturing infrastructure serves as a springboard for MedTech startups and scale-ups. Combined with technical and regulatory expertise, it significantly shortens the time to market for companies transforming the healthcare landscape.

Photo credit: STENTiT
STENTiT: Founded in 2017 as a spin-off from TU/e, STENTIT is a medical device company developing regenerative endovascular implants, specifically bioresorbable devices that trigger natural healing responses by circulating blood cells.
Resorbable Fibrillated Scaffold (RFS) is a temporary vascular support structure capable of reconstructing arteries to provide better clinical outcomes. The device is designed to restore blood flow and promote natural arterial healing, representing what STENTiT calls “the next generation in stent technology.”
STENTiT enrolled its first patient in the VITAL-IT 1 clinical trial, successfully implanting the RFS device. The early-stage clinical study is designed to test the safety and performance of the RFS device in up to ten patients with chronic limb-threatening ischemia (CLTI), specifically targeting severe blood flow problems in the lower leg.
STENTiT is supported by the European Innovation Council’s EIC Accelerator program and was selected for the 2025 MedTech Innovator cohort.

Photo credit: VIVOLTA
VIVOLTA is a contract development and manufacturing organization (CDMO) specializing in electrospinning technology for medical devices and drug delivery systems. VIVOLTA develops and manufactures electrospun nanofiber-based medical implants and devices that mimic the human extracellular matrix to guide the body to heal itself.
VIVOLTA represents the perfect partner in the MedTech ecosystem and will relocate to HTCE in 2026.
Sports, vitality and health companies form an essential part of the MedTech ecosystem at High Tech Campus Eindhoven, bridging the gap between clinical care and everyday wellness through preventive health solutions and performance optimization.
By focusing on keeping people healthy and active rather than solely treating illness, these companies complement HTCE’s diagnostic and therapeutic innovations, contributing to a holistic approach that spans the complete health spectrum from prevention to recovery.
The activities of these companies in a nutshell:
ARION/ATOGEAR: Insights through data and AI into the field of sports.
Arjo: Supplier of medical equipment
Maurten: Sports nutrition
Ocuma: AI-powered safety for public swimming pools
Shimano Europe: Cycling components, fishing tackle and rowing equipment
Sparckel: Daylight lamp
VitalWear: Optical fiber sensing technology in textiles for non-invasive medical applications
Workplace Vitality Hub: Innovation Hub at HTCE on Vitality by Fontys, imec, TNO, TU/e, HTCE
Breakthrough medical and biomedical technologies begin at research institutes, and HTCE is home to some of the world's leading innovation centers in health technology development.
These institutes serve as the critical foundation of the Campus's MedTech ecosystem, transforming fundamental scientific discoveries into practical applications through expertise in flexible electronics, wearable sensors, advanced diagnostics and integrated photonics.
By fostering collaboration between academia, industry and startups and actively spinning out new companies, these research powerhouses accelerate the journey from laboratory concepts to commercially viable medical solutions that improve patient care.
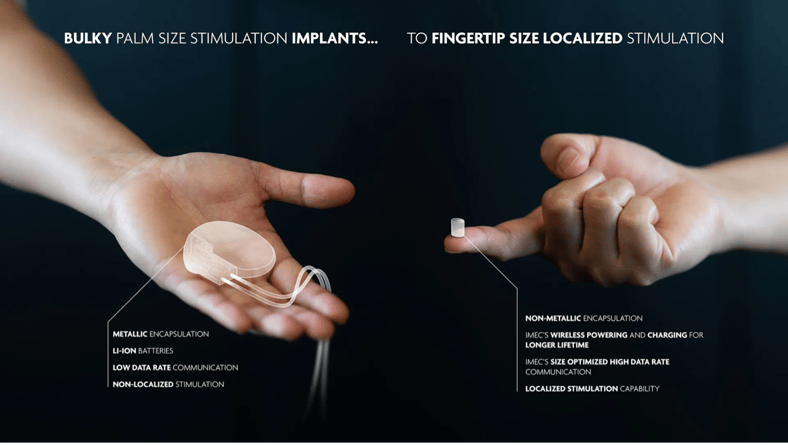
Photo credit: Holst Centre
Holst Centre is a unique research and innovation center specializing in health technologies and flexible and wireless electronics, powered by the shared expertise of imec and TNO. With the mission to improve healthcare and make it make it more affordable at the same time, Holst Center develops wearable health solutions such as smart patches for diagnostics and continuous health monitoring, integrating sensors and electronics into flexible, comfortable devices for vital sign tracking and medical applications.
It also develops thin-film and flexible electronics technologies, which enable advanced medical imaging modalities and large-area sensors that improve diagnostics and image-guided therapies. AIKON Health and Touchwaves use technology developed at Holst Centre.

Photo credit: imec
imec is a key player in the Brainport and High Tech Campus MedTech community. Together with TNO, imec created the Holst Centre, an independent R&D center focused on wireless sensor technologies, flexible electronics and wearable health solutions.
imec leads advanced health tech innovation in diagnostics, wearable health and personalized care and supports development of pioneering MedTech devices and digital health solutions within our vibrant innovation ecosystem at Campus.
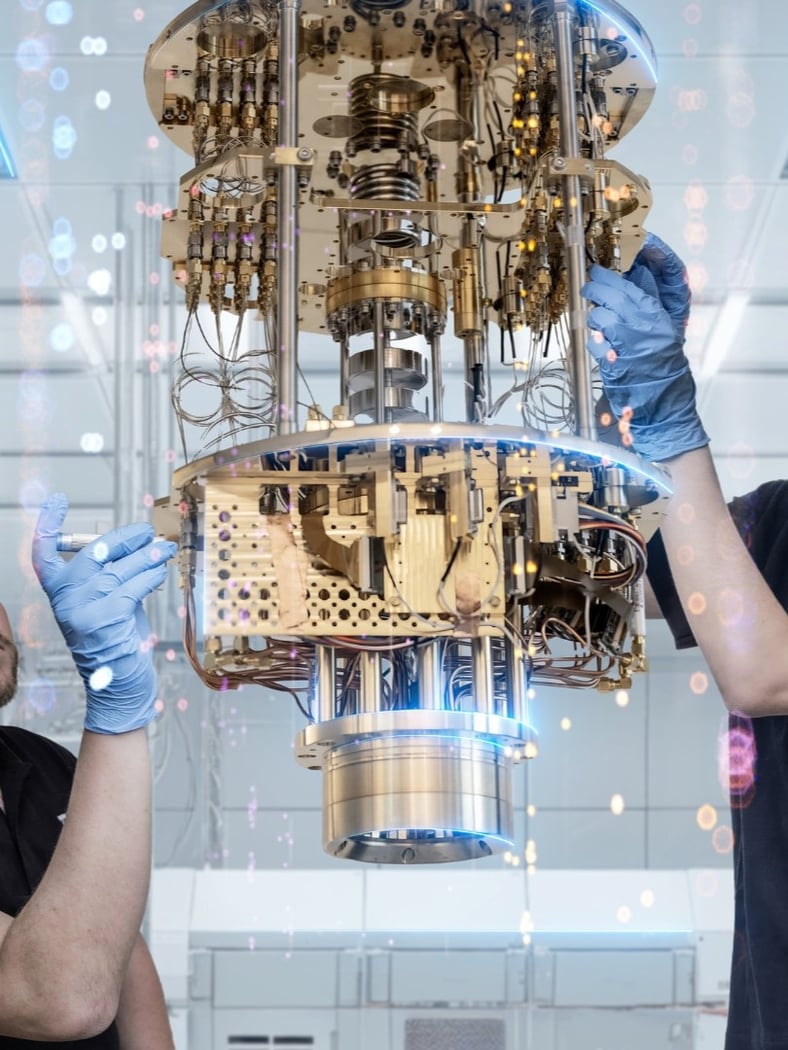
Photo credit: TNO
TNO is a key research institute driving technological innovation in the MedTech sector. TNO develops advanced medical devices, biosensors and tools for diagnostics with integrated photonics and optics technology, crucial for advanced medical diagnostics and devices.
TNO coordinates research within large-scale collaborations such as Eindhoven MedTech Innovation Center (e/MTIC) and public-private partnerships within initiatives, such as MedTechNL, to accelerate innovations from lab to market and build a strong, sustainable healthcare sector.
Its technologies, which range from tissue optics modeling to wearable health sensors, serve local startups and established players, providing advanced solutions for medical imaging, point-of-care diagnostics and personalized health monitoring.
TNO helps clear the path for new MedTech solutions to advance from research and development to commercialization and market implementation, and TNO Ventures invests in its spinouts. The TNO Ventures program brings together stakeholders to bring innovations to life and make a direct impact on society. There are nine TNO MedTech spin-offs, including AIKON Health and Aiosyn, a LUMO Labs portfolio company.
Stay tuned for the next deep dive edition on the MedTech & BioTech cluster at HTCE! The next edition focuses on all supporting service and facility providers as well as ecosystem partners.
Download the entire overview of 70+ MedTech & BioTech companies at HTCE.